Molnupiravir Ivermectin : F2yzmmdnftw77m
No one has to wait. They include ivermectins original manufacturer Merck which has an antiviral compound molnupiravir in Phase 3 clinical trials for COVID-19.

Anti Viral Pill Molnupiravir Shows Promise Against Covid Other Viruses Youtube
Merck lost the patent years ago.

Molnupiravir ivermectin. Drugmaker tested its antiviral against nasal swab samples taken from. However the current data is considered inconclusive. Moreover the New Jersey-based pharmaceutical company was compelled by a.
The creator of Molnupiravir is the same company that made the controversial ivermectin that is used to treat the parasitic infections of three hundred million people. Another expensive and rushed to market proprietary drug through the EAU. Food and Drug Administration FDA to treat parasite type diseases like onchocerciasis and lymphatic filariasis.
That might explain the companys recent statement claiming that there is no scientific basis whatsoever for a potential therapeutic effect of ivermectin against COVID-19. As you read the news reports on a new anti-viral pill to battle COVID-19 from Merck called Molnupiravir keep in mind Merck is the pharma company that owns the anti-viral pill Ivermectin. Unfortunately Merck has already sold a Billion dollars of doses to the US govt.
However if ivermectin was officially recognized as an effective treatment it would legally prevent molnupiravirs EUA until it passes trials and thus delay or endanger the 12 billion deal. According to results of their studies around 7 of people who took Molnupiravir were hospitalized or died from COVID compared to 14 who. It just needs to be recommended to treat COVID-19.
Oct 1 Reuters Positive clinical trial results for Merck Cos experimental antiviral COVID-19 pill reverberated through the healthcare sector on Friday sending the drugmakers. Molnupiravir looks to be a protease inhibitor blocking the cleaving of translated viral polypeptides into functional proteins required for SARS-CoV-2 to replicate successfully. Anyone can make ivermectin.
Ivermectin doesnt need an EUA because it passed trials in 1986. Merck has stopped clinical trials of their Molnupiravir on grounds that the results are so profound that it should be authorized for emergency use. So a new expensive exclusive and proprietary therapeutic was needed.
The creator of Molnupiravir is the same company that made the controversial drug ivermectin which is used to treat parasitic infections for 300 million people. Did Merck Publicly Smear Ivermectin to Make Way for Their New Drug Molnupiravir. Ivermectin has received approval from the US.
Rule 2 does apply throughout the rest of this thread. The reason is that Molnupiravir is reportedly very similar to Ivermectin. No money to be made.
Rule 2 does not apply when replying to this stickied comment. Data shows that the drug is most effective when given early in the course of infection Merck said. Molnupiravir ist eines von vielen Mitteln die aktuell gegen das Coronavirus entwickelt werden.
Because we have such a drug right now. Molnupiravir instead targets the viral polymerase an enzyme needed for the virus to make copies of itself. They include ivermectins original manufacturer Merck which has an antiviral compound molnupiravir in Phase 3 clinical trials for COVID-19.
KENILWORTH NJ On Friday multinational pharmaceutical. That might explain the companys recent statement claiming that there is no scientific basis whatsoever for a potential therapeutic effect of ivermectin against COVID-19. News On AlhimarCom How to watch addams family 2 How to watch venom let there be carnage How to watch venom 2 How to get rid of boxelder bugs How to block spam calls How to draw a pumpkin How to win powerball How to play powerball How to factory reset iphone How to get rid of stink bugs How to cook salmon How to write a check How to.
Please contact the moderators of this subreddit if you have any. By the laws of fiduciary duty to shareholders Merck cannot support Ivermectin over Molnupirvir as evidenced by its official statement on the Merck website that Ivermectin. I can see using them in conjunction to good effect for those infected.
Merck is focusing on molnupiravir. An aggravating factor is the fact that molnupiravir EIDD-2801. Its called ivermectin and it is preventing the disease and saving lives from Covid-19 in every country.
KENILORTH NJ Multinational drug company Merck NYSEMRK known outside the United States and Canada. Please keep any meta discussion directed at specific users mods or rconspiracy in general in this comment chain only. Ivermectin is off-patent and zero profits.
Prophylactic means to prevent infection are also out there you just have to look for the articles some of which data to 2010. Molnupiravir Merck COVIDtreatmentHi everyone. Is New COVID 19 Drug Molnupiravir Better than Ivermectin.
TrialSite Staff June 7 2021. Anyone can make ivermectin. What is particularly disturbing is that it appears that molnupiravir contains some of the same molecular qualities as ivermectin which makes you wonder if Merck knows that ivermectin is effective and just sought a more expensive drug that could be marketed as exclusive and new for COVID thereby justifying another budget blowout by Washington.
I am a bot and this action was performed automatically. The study concluded that apparent safety and low cost suggest that Ivermectin is likely to have a significant impact on the SARS-CoV-2 pandemic globally So why isnt Merck just pushing Ivermectin instead. Not even for a minute.
Merck Tests Molnupiravir an Investigational Product at the Lung Center of the Philippines While Large Ivermectin Trial Runs in Parallel. You are not going to make all of humanity wait for your brand spanking new antiviral drug molnupiravir that you have touted as the answer to the worlds prayers for Covid-19. Early in the pandemic ivermectin was thought to help treat COVID-19 however current data are inconclusive.
Can Molnupiravir help in Long HaulersMolnupiravir originally called EIDD-2801 was developed by t. Molnupiravir could fill the role that many are currently incorrectly using ivermectin for. This is why they dropped their vaxx research months ago and poured all their research into therapeutics.
While the Philippines recently announced a large national ivermectin trial targeting early-onset COVID-19 another commercial study seeks to accelerate sufficient safety. At the beginning of the pandemic ivermectin was thought to help treat COVID-19. It is designed to work by introducing errors into the genetic code of the virus.
Of its Covid treatment drug Molnupiravir. Lets look at a promising new oral drug Molnupiravir for treating mild to moderate COVID this week.

Molnupiravir Last Of The Small Molecule Coronavirus Hopes Science Aaas
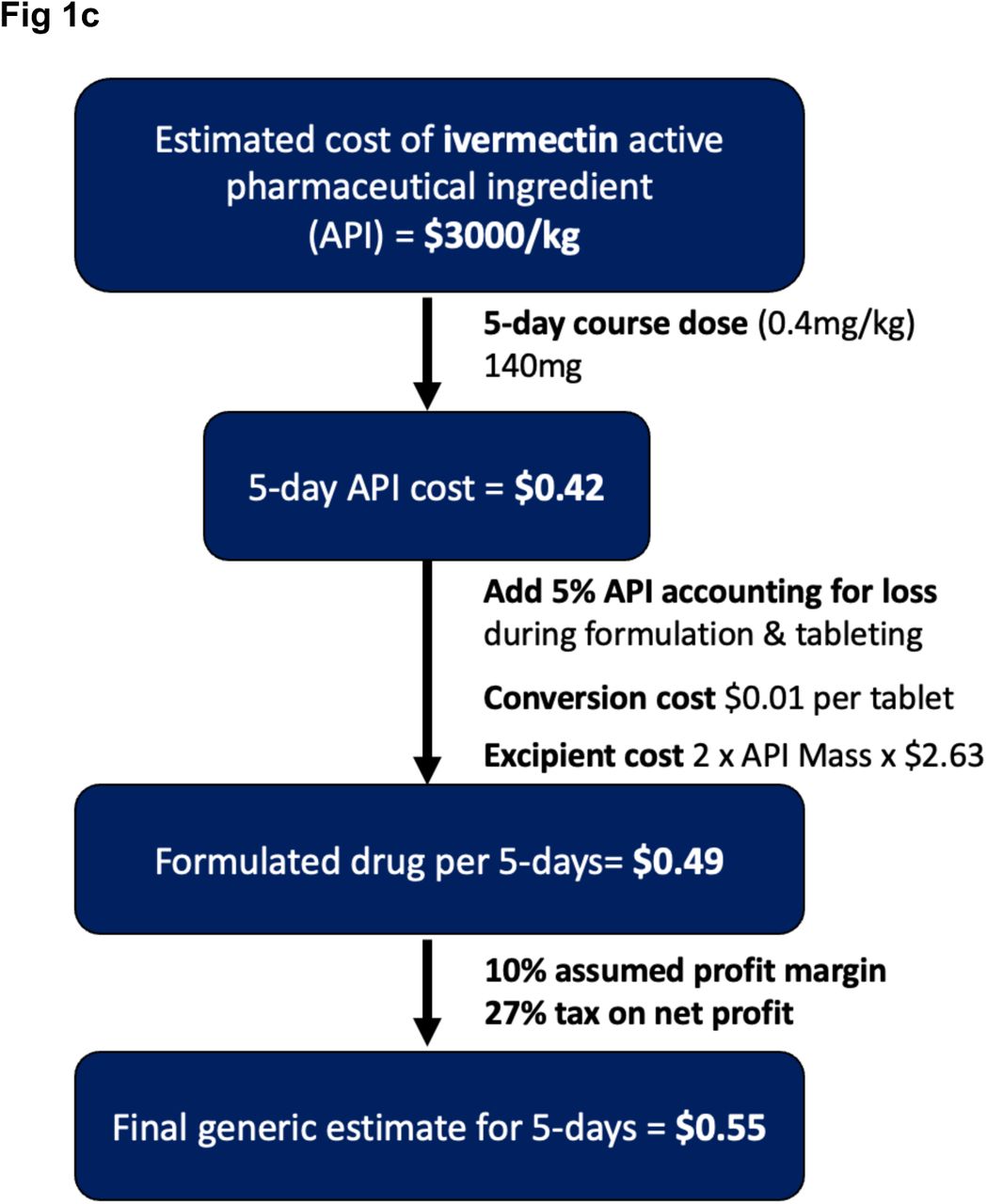
Minimum Manufacturing Costs National Prices And Estimated Global Availability Of New Repurposed Therapies For Covid 19 Medrxiv

Merck Tests Molnupiravir An Investigational Product At The Lung Center Of The Philippines While Large Ivermectin Trial Runs In Parallel

Unitaid Statement Regarding Ivermectin As A Potential Covid 19 Treatment Unitaid
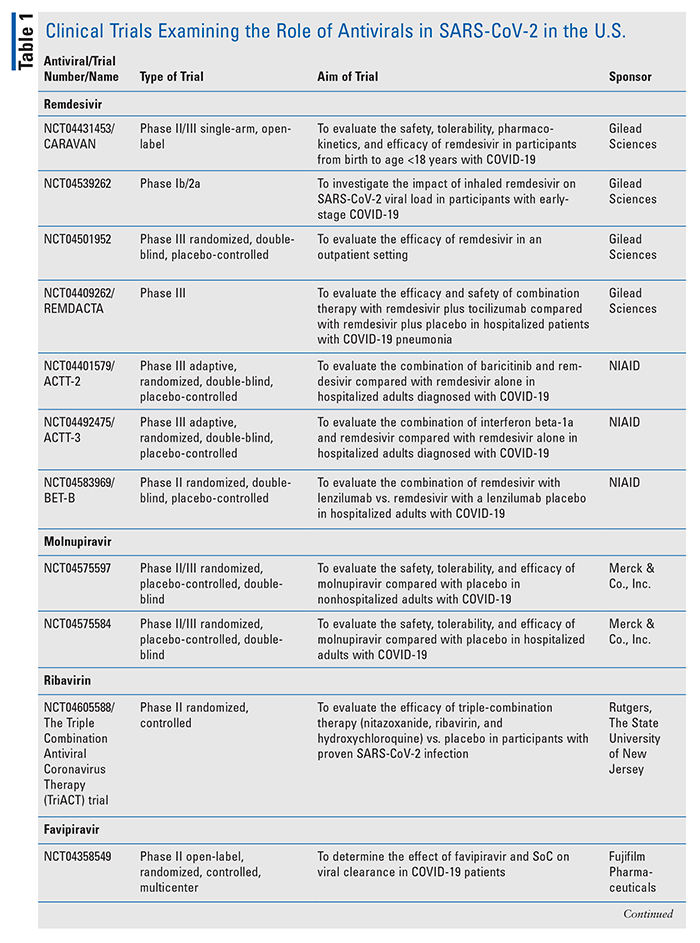
Investigational Antivirals For Managing Covid 19
Lung Center Qmmc Call For Participants In Molnupiravir Trials Vs Covid 19
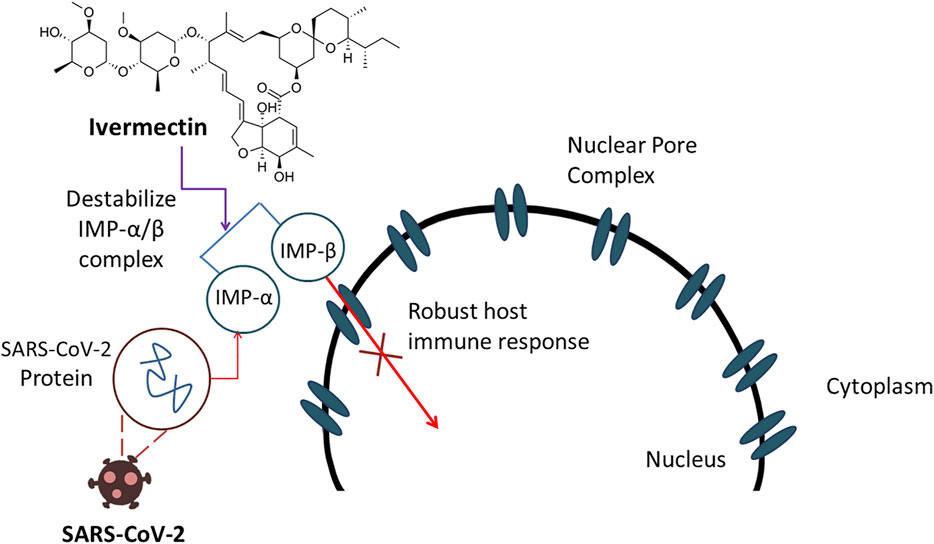
Frontiers Therapeutic Effectiveness And Safety Of Repurposing Drugs For The Treatment Of Covid 19 Position Standing In 2021 Pharmacology

Merck Mrk Molnupiravir Pill Could Change The Fight Against Covid Bloomberg

Scientists Hope They Re Closing In On A Cure For Covid 19 Pbs Newshour

How Molnupiravir Moved To The Head Of The Covid Pill Pack Medpage Today

The Biden Administration Investing 3 2 Billion To Find New Antiviral Drugs The Washington Post

New Antiviral Oral Drug Molnupiravir Completely Suppresses Coronavirus Transmission Within 24 Hours In Ferrets Claims Study Health News Firstpost

:no_upscale()/cdn.vox-cdn.com/uploads/chorus_image/image/69938987/AP21274373443876_copy.0.jpg)